Powder: -20°C for 3 years | In solvent: -80°C for 1 year
Metformin hydrochloride [1115-70-4]
Cat# T0740-50mg
Size : 50mg
Marca : TargetMol
Metformin hydrochloride
Catalog No. T0740 CAS 1115-70-4
Synonyms: Metformin HCl, 1, 1-Dimethylbiguanide hydrochloride, 1,1-Dimethylbiguanide hydrochloride
Metformin hydrochloride (1,1-Dimethylbiguanide hydrochloride) , a widely used anti-diabetic drug, has a potential function as an anti-Y medicine. It inhibits the proliferation of a variety of Y cells including colon, prostate, and etc.
All products from TargetMol are for Research Use Only. Not for Human or Veterinary or Therapeutic Use.
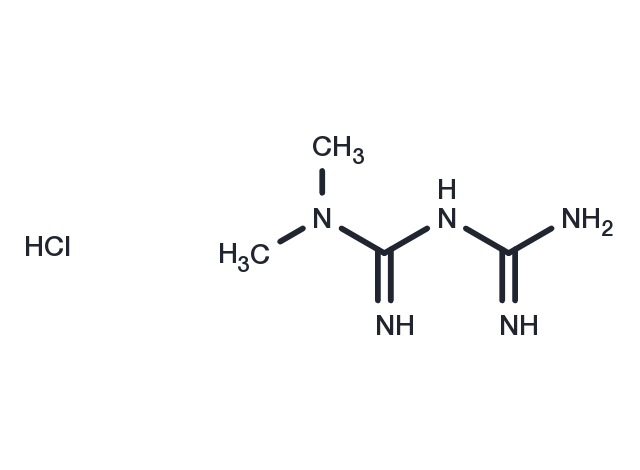
Metformin hydrochloride, CAS 1115-70-4
| Description | Metformin hydrochloride (1,1-Dimethylbiguanide hydrochloride) , a widely used anti-diabetic drug, has a potential function as an anti-Y medicine. It inhibits the proliferation of a variety of Y cells including colon, prostate, and etc. |
| In vitro | In primary cultured hepatocytes from both rats and humans, metformin activated AMPK in a concentration- and time-dependent manner. rat hepatocytes were incubated with 10 μM or 20 μM metformin for 39 hours. Both 10 μM and 20 μM metformin produced significant AMPK activation [1]. Metformin inhibited proliferation of ESCs in a concentration-dependent manner. The IC50 was 2.45?mmol/l for adenomyotic endometrial stroma cells (A-ESCs) and 7.87?mmol/l for normal endometrial stromal cells (N-ESCs) [2]. Metformin selectively kills cancer stem cells (CSCs) and, as such, acts together with chemotherapy to inhibit tumor growth and prolong remission in mouse xenografts. In CSCs, metformin significantly inhibits expression of a variety of inflammatory genes, Lin28B gene expression, and VEGF protein expression [3]. |
| In vivo | Hepatic fatty acid oxidation was induced in metformin-treated rats. Furthermore, metformin treatment produced significant decreases in hepatic expression of mRNAs for SREBP-1, FAS, and S14 [1]. Crl:CD(SD) rats were administered metformin at 0, 200, 600, 900 or 1200 mg/kg/day by oral gavage for 13 weeks. Administration of > or =900 mg/kg/day resulted in moribundity/mortality and clinical signs of toxicity. Other adverse findings included increased incidence of minimal necrosis with minimal to slight inflammation of the parotid salivary gland for males given 1200 mg/kg/day, body weight loss and clinical signs in rats given > or =600 mg/kg/day [4]. |
| Cell Research | Hepatocytes were isolated from male Sprague Dawley (SD) rats by collagenase digestion. For the AMPK assay, cells were seeded in six-well plates at 1.5 × 10^6 cells/well in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, 10% FBS, 100 nM insulin, 100 nM dexamethasone, and 5 μg/ml transferrin for 4 hours. Cells were then cultured in serum-free DMEM for 16 hours followed by treatment for 1 hour or 7 hours with control medium, 5-aminoimidazole carboxamide riboside (AICAR), or metformin at concentrations indicated. For a 39-hour treatment, cells for both control and metformin (10 or 20 μM) groups were cultured in DMEM plus 5% FBS and 100 nM insulin, and the fresh control and metformin-containing medium were replaced every 12 hours (last medium change was 3 hours before harvest). After treatment, the cells were directly lysed in digitonin-containing and phosphatase inhibitor–containing buffer A, followed by precipitation with ammonium sulfate at 35% saturation. AMPK activity was determined by measurement of phosphorylation of a synthetic peptide substrate, SAMS (HMRSAMSGLHLVKRR). For ACC assay, the 35% ammonium sulfate precipitate from digitonin-lysed hepatocytes (4 μg each) was used for determination of ACC activity via 14CO2 fixation in the presence of 20 mM citrate as done previously. For fatty acid oxidation, the oxidation of 14C-oleate to acid-soluble products was performed as done previously, but in medium M199 in the absence of albumin [1]. |
| Animal Research | Oral gavage was used to administer 1 ml of metformin (100 mg/ml) or water alone to male SD rats (300–350 g, n = 7–8). Rats were treated once or twice a day for 5 days. Rats were starved for 20 hours and then re-fed for 2 hours before the final dose; 4 hours after the final dose, the animals were anesthetized and livers rapidly removed by freeze clamping followed by blood withdrawal. RNA was prepared from the freeze-clamped liver by RNA isolation reagent. Nuclear extracts were prepared from a pool of seven rat livers. Glucose levels were determined using the standard glucose oxidase assay kit; β-hydroxybutyrate concentrations were assayed by measuring the reduction of NAD to NADH with a standard assay kit. FFA levels were measured with the assay kit [1]. MCF10A-ER-Src cells (5 × 10^6) were injected into the right flank of 18 female nu/nu mice, all of which developed tumors in 10 d with a size of ~100 mm^3. The mice were randomly distributed into six groups (three mice/group) that were untreated or treated by intratumoral injections every 5 d (four cycles) with 1 mg/kg or 4 mg/kg doxorubicin, 200 μg/mL metformin (diluted in the drinking water), or the combination. In another experiment, LNCaP and DU145 prostate cancer cells (5 × 10^6) were injected into the right flank of 12 female nu/nu mice, all of which developed tumors in 10 d with a size of ~75 mm^3. The mice were randomly distributed into four groups that were untreated or treated by intratumoral injections every 5 d (four cycles) with 4 mg/kg doxorubicin and/or 200 μg/mL metformin. In another experiment, A375 and MDA-MB-435 melanoma cells (7 × 10^6) were injected into the right flank of 12 female nu/nu mice, all of which developed tumors in 10 d with a size of ~50 mm3. The mice were randomly distributed into four groups that were untreated or treated by intratumoral injections every 5 d (four cycles) with 10 mg/kg cisplatin and/or 200 μg/mL metformin.Finally, SNU-449 liver cancer cells (10^7) were injected into the right flank of 12 female nu/nu mice, all of which developed tumors in 10 d with a size of ~50 mm^3. The mice were randomly distributed into four groups that were untreated or treated by intratumoral injections every 5 d (four cycles) with 10 mg/kg cisplatin and/or 200 μg/mL metformin. Tumor volume (mean ± SD) was measured at various times after the initial injection [3]. |
| Synonyms | Metformin HCl, 1, 1-Dimethylbiguanide hydrochloride, 1,1-Dimethylbiguanide hydrochloride |
| Molecular Weight | 165.63 |
| Formula | C4H12ClN5 |
| CAS No. | 1115-70-4 |
Storage
Solubility Information
H2O: 193.21mM
DMSO: 15 mg/mL (90.56 mM), Sonication is recommended.
 References and Literature
References and Literature
1. Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001 Oct;108(8):1167-74. 2. Xue J, et al. Metformin inhibits growth of eutopic stromal cells from adenomyotic endometrium via AMPK activation and subsequent inhibition of AKT phosphorylation: a possible role in the treatment of adenomyosis. Reproduction. 2013 Aug 21;146(4):397-406. 3. Hirsch HA, et al. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):972-7. 4. Quaile MP, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010 Mar 15;243(3):340-7. 5. Liang C, Sun R, Xu Y, et al. Effect of the Abnormal Expression of BMP-4 in the Blood of Diabetic Patients on the Osteogenic Differentiation Potential of Alveolar BMSCs and the Rescue Effect of Metformin: A Bioinformatics-Based Study[J]. BioMed Research International. 2020, 2020. 6. He Y, Xu K, Wang Y, et al. AMPK as a potential pharmacological target for alleviating LPS-induced acute lung injury partly via NLRC4 inflammasome pathway inhibition[J]. Experimental Gerontology. 2019: 110661. 7. Zhu X, Shen W, Liu Z, et al. Effect of metformin on cardiac metabolism and longevity in aged female mice[J]. Frontiers in Cell and Developmental Biology. 2020, 8. 8. Jia Y, Cui R, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway[J]. Redox Biology. 2020: 101534. 9. Zhao H, Li T, Wang K, et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca 2+ entry to promote mitotic progression[J]. Nature cell biology. 2019, 21(4): 476-486. 10. Tang Y, Fang G, Guo F, et al. Selective Inhibition of STRN3-Containing PP2A Phosphatase Restores Hippo Tumor-Suppressor Activity in Gastric Cancer[J]. Cancer Cell. 2020, 38(1): 115-128. e9.
 Citations
Citations
1. Tang Y, Fang G, Guo F, et al. Selective Inhibition of STRN3-Containing PP2A Phosphatase Restores Hippo Tumor-Suppressor Activity in Gastric Cancer. Cancer Cell. 2020, 38(1): 115-128. e9. 2. Zhao H, Li T, Wang K, et al. AMPK-mediated activation of MCU stimulates mitochondrial Ca 2+ entry to promote mitotic progression. Nature cell biology. 2019, 21(4): 476-486. 3. Jia Y, Cui R, Wang C, et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biology. 2020: 101534. 4. Zhu X, Shen W, Liu Z, et al. Effect of metformin on cardiac metabolism and longevity in aged female mice. Frontiers in Cell and Developmental Biology. 2021 Jan 26;8:626011. doi: 10.3389/fcell.2020.626011. eCollection 2020. 5. He Y, Xu K, Wang Y, et al. AMPK as a potential pharmacological target for alleviating LPS-induced acute lung injury partly via NLRC4 inflammasome pathway inhibition. Experimental Gerontology. 2019: 110661. 6. Liang C, Sun R, Xu Y, et al. Effect of the Abnormal Expression of BMP-4 in the Blood of Diabetic Patients on the Osteogenic Differentiation Potential of Alveolar BMSCs and the Rescue Effect of Metformin: A Bioinformatics-Based Study. BioMed Research International. 2020, 2020 7. Cai Z, Wu X, Song Z, et al.Metformin potentiates nephrotoxicity by promoting NETosis in response to renal ferroptosis.Cell Discovery.2023, 9(1): 104.



