Enhanced Plasmid Production: Achieving a 21-Fold Increase in Plasmids

In this work, a plasmid production process was performed comparing conventional Erlenmeyer flasks with LB medium and the Thomson’s Ultra Yield® system. E. coli cells were cultivated in both systems in an orbital incubator shaker (Multitron, INFORS HT), bacterial growth was monitored, and the plasmids were purified at the end of cultivation. Subsequently, the quality of the isolated plasmids was analyzed by HPLC. The combination of the Ultra Yield® flasks with the enriched Plasmid+® medium and the AirOtop® enhanced seal led to higher cell densities and a 21-fold higher amount of plasmid than in Erlenmeyer shake flasks with LB medium.

Introduction
The demand for plasmid DNA (pDNA) has increased over the last few years as a result of the high demand for gene therapies and DNA vaccinations (pDNA is commonly used because of its high safety). Therefore, an increased production of pDNA with a cost-effective, reproducible, and reliable purification and quality control system is highly demanded. Plasmids are typically produced in Escherichia coli (E. coli) cells and subsequently isolated by a series of purification steps. Although E. coli mainly produces the more compact supercoiled (SC) plasmid DNA (pDNA) isoform, open circular (OC), nicked, linear and denatured pDNA isoforms are usually also present. The occurrence of different isoforms can be caused by conformational changes which occur in the bacterial host and during biomass processing (e.g. cell lysis) and plasmid purification steps [1]. Several lines of evidence indicate that high SC levels are required for eliciting an effective immune response and ultimately, protection from infections [2,3]. Furthermore, the SC pDNA isoform is the desired isoform for transfection as it allows a higher transfection efficiency due to its more compact packing compared to the OC or linear variants [4–6].
Anion-exchange chromatography (AEC) is a common method to purify SC pDNA from other plasmid isoforms and to remove present impurities derived from the host organism. Ideally, the upstream production process already delivers predominantly high-quality SC pDNA.
Materials and Methods
Transformation and cultivation
The pCMV3-GFP plasmid (6883 bp) containing an ampicillin resistance gene (Sino Biological) was transformed into NEB 5-alpha competent E. coli cells (New England Biolabs) according to the manufacturer’s protocol. The transformation mix was plated on a selection plate (LB agar with 100 µg/mL ampicillin) and incubated overnight at 37°C. A single colony was chosen from the selection plate and an overnight culture was prepared in a 500 mL unbaffled glass Erlenmeyer flasks containing 50 mL of LB medium (tryptone 10 g/L, Yeast extract 5 g/L, NaCl 10 g/L, pH 7.0 ±0.2) with 100 µg/mL ampicillin. The flask was sealed with aluminium foil and was incubated at 37°C and 180 min –1 in an orbital incubator shaker with Sticky Stuff adhesive mats (INFORS HT Multitron Incubator Shaker, 25 mm shaking throw) overnight (at least 18 hours). For the main culture, triplicates of 250 mL unbaffled glass Erlenmeyer flasks containing 25 mL of LB with 100 µg/mL ampicillin were prepared, which is considered a standard cultivation design.
Triplicates of 250 mL Ultra Yield® flasks (Thomson, P/N: 931144) containing 100 mL of Plasmid+® medium (Thomson, P/N: 446300) with 100 µg/mL ampicillin and 0.02% of Antifoam 204 (Sigma-Aldrich) were prepared. Both the standard cultivation system and Thomson cultivation system were inoculated from the overnight culture, with a starting OD600nm of 0.2. The Ultra Yield® flasks were sealed with the AirOtop® Enhanced Seal (Thomson, P/N: 899423) and the Erlenmeyer flasks with aluminum foil. Flasks were incubated at 37°C at 350 min –1 or 180 min –1 in the INFORS HT Multitron Incubator Shaker (25 mm shaking throw) with flask clamps. Note that Sticky Stuff adhesive mats are not suitable for 350 min –1 and the use of clamps is mandatory. Cultures were grown for at least 24 hours and samples were taken at regular intervals to measure OD600nm over time.
DNA purification
The PureYield™ Plasmid Miniprep System (Promega) was used for plasmid purification. LB cell suspension was used undiluted, Thomson cell suspensions were diluted 9-fold to approximately match the OD value of LB samples. 600 µL was sampled of each cell suspension which was then centrifuged, and the pellet was resuspended in 600 µL nuclease free water. All other subsequent steps were carried out in accordance with the PureYield™ Plasmid Miniprep System quick protocol FB093 for kit A1223 or A1222. The DNA purification was performed by the centrifugation method. The plasmids were eluted with nuclease free water provided with the kit. The DNA concentration and purity of the eluted samples were measured at 230, 260 and 280 nm with a NanoDrop™ 2000/2000c Spectrophotometers (Thermo Fisher Scientific).
Plasmid analysis using HPLC
For plasmid analysis an HPLC 1100 Series (Agilent) with DAD detector 1100 Series (Agilent, G1315B) at 260 nm was used. Samples were measured on a BioPro IEX QF, 100 x 4.6 mm, 5 µm particle size column (YMC, QF00S05-1046WP). 5 µL of each sample was injected and measured in triplicates. The column temperature was set at 35°C. Mobile phase A (20 mM Tris-HCl, pH 7.4) and mobile phase B (20 mM Tris-HCl, 1 M NaCl, pH 7.4) were used to elute the sample in a gradient method with a constant flow of 0.5 mL/min. The gradient was performed from minute 0 until minute 0.7 with mobile phase A (MPA) at 25% and mobile phase B (MPB) at 75%. Starting at minute 0.7 the gradient was applied and the composition from 75% MPB was changed to 100% during 20 minute until minute 20.7 and was kept at 100% until minute 24. At minute 24.01 the composition was changed to 25% MPA and 75% MPB until minute 30 (end of the injection).
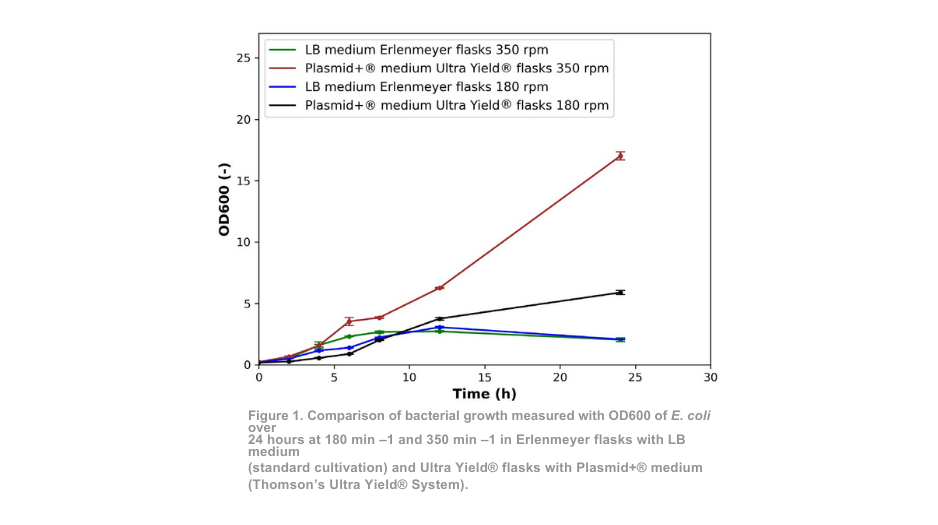
Bacterial growth in Erlenmeyer flasks with LB medium (standard cultivation) at 350 min–1 and Ultra Yield® flasks with Plasmid+® medium at 350 min –1 exhibited similar patterns up to 4 hours of cultivation, as depicted in Figure 1. For cultivation at 180 min –1 the similarities are seen up until 8 hours. In the case of bacterial growth in Ultra Yield® flasks, the initial growth rate is slightly slower during the first few hours compared to the standard cultivation.
However, a distinct contrast between the two cultivation conditions becomes evident after 4 hours (350 min –1) and 8 hours (180 min –1). For the standard cultivation approach, the optical density is reduced compared to the measurement at 12 hours, indicating that the number of viable cells is already declining at this timepoint. At the end of the cultivation period, the OD values of the Ultra Yield® system maintained at 180 min–1 and 350 min–1 exceeded that of the standard cultivation system by more than 2-fold and 7-fold respectively.
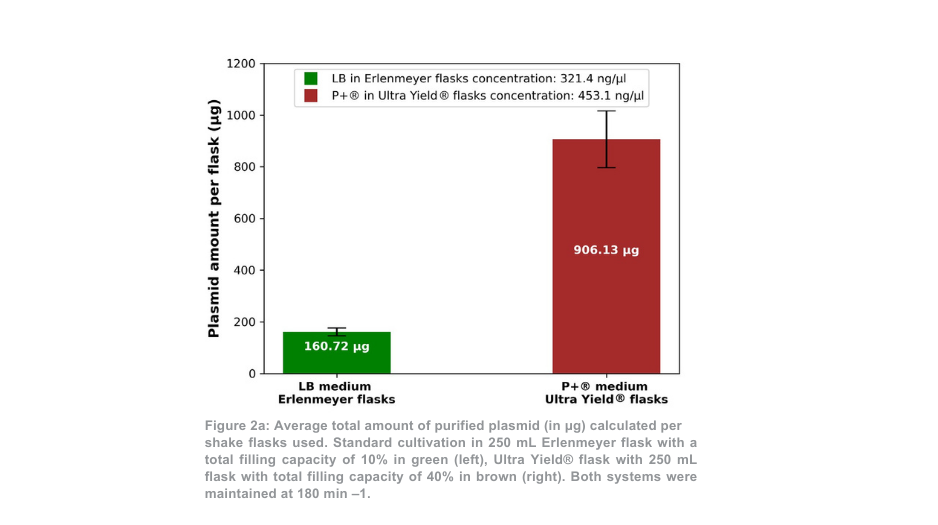
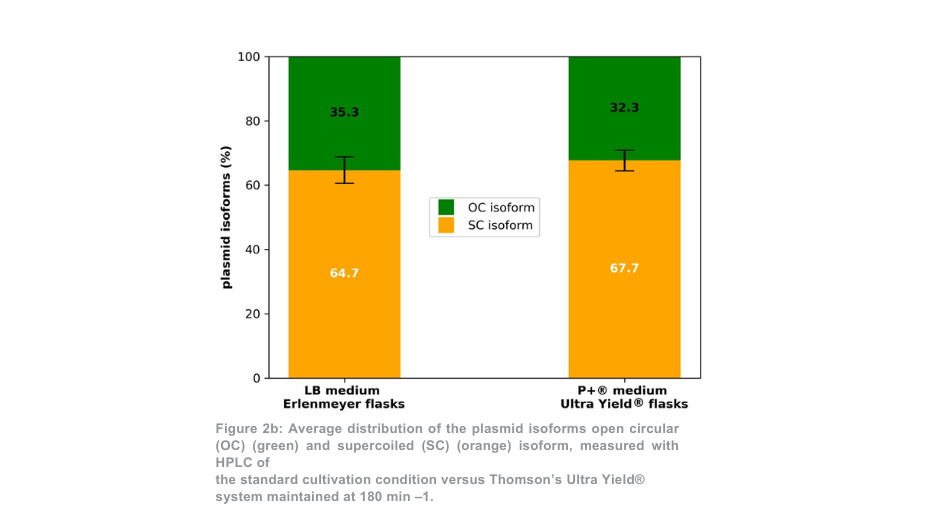
After plasmid purification, the total plasmid yield for the 180 min –1 condition was more than 5-fold higher per Thomson flask as compared with the Erlenmeyer flasks (Figure 2a). When the volume difference between flasks was accounted for, the plasmid yield was 1.4-fold higher with the Thomson cultivation system versus the standard cultivation system. The proportion of supercoiled plasmid to open coiled plasmid (Figure 2b) was similar between the Thomson cultivation system (67.7% supercoiled) and the standard cultivation system (64.7% supercoiled).
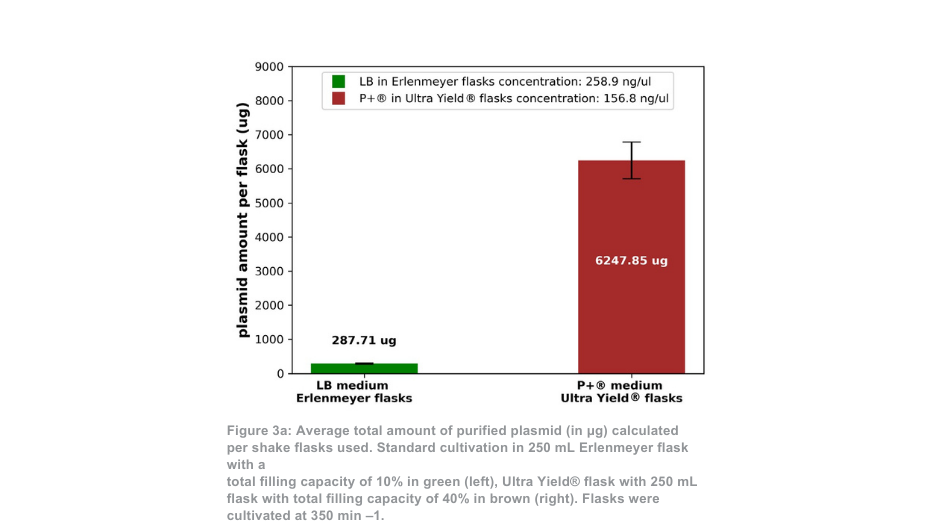
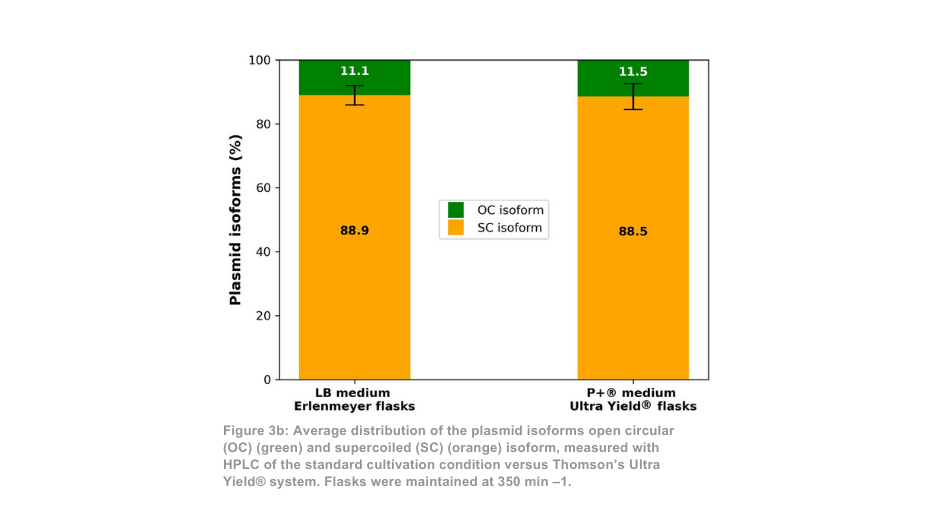
The difference between the Thomson cultivation system and standard cultivation system is more pronounced when maintained at 350 min –1. In this case, the total amount of purified plasmid per flask is around 21-fold higher with Thomson’s Ultra Yield® system compared to the standard cultivation condition, or roughly 5-fold higher when the volume difference is accounted for (Figure 3a). The proportion of supercoiled plasmid to open coiled plasmid (Figure 3b) was again similar between the Thomson cultivation system (88.5% supercoiled) and the standard cultivation system (88.9% supercoiled).
Conclusion
The data demonstrates that common shaking speeds of 180 min –1 (25 mm orbit) are not enough to support optimal cell growth in the Thomson Ultra Yield® flasks. A high shaking speed of 350 min –1 provides a 3-fold increase of biomass yield, compared to 180 min –1. With better cell growth, higher pDNA production was achieved. The success of this application relies heavily on maintaining a high agitation speed to reach effective mixing and gas transfer, leading to higher yields. As agitation speeds increase from 180 min –1 to 350 min –1, there is a 4-fold rise in the kinetic energy required to shake the surface effectively. It is critical that the shaker’s load limit can safely accommodate the flasks at high speeds. The INFORS HT Multitron Incubator Shaker can hold 45 x 250 mL flasks, with a media and culture mass alone totaling 4.5 kilograms. Considering the additional mass of the tray and flask clamps, the shaker’s minimum load limit should exceed 12 kilograms. With larger flasks, it is possible to load more than 10 L of media into the shaker, making the shaker’s physical load limit and interior dimensions extremely important. All of these factors emphasize the necessity of a robust shaker, like the Multitron Incubator Shaker, to fully realize the benefits of this process.
The combination of the Ultra Yield® flasks with the enriched Plasmid+® medium and the AirOtop® enhanced seal led to higher cell densities and a 21-fold higher amount of plasmid than in Erlenmeyer shake flasks with LB medium. The overall higher yield of plasmids also results in a higher amount of the supercoiled (SC) plasmid isoform which is desired for transfection of mammalian cells [4,5]. Considering the total cost of ownership of a plasmid production process in the lab, the use of Thomson Ultra Yield® flasks, Plasmid+® medium in combination with a powerful incubator shaker at 350 min –1 is less expensive in plasmid mg/L compared to LB cultivation in glass Erlenmeyers. Investing in a good shaker to reach higher speeds, and Thomson System is advantageous.
Use AIROTOP® Seal/Vented Screw Cap Media mL/Flask Shaker Speed (RPM)
| Code | Description | Use AIROTOP® Seal/Vented Screw Cap | Media mL/Flask | Shaker Speed (RPM) |
| 931147 | 125mL Ultra Yield® Flasks 50/CS - Sterile | 899421 / 899109 | 35-50 mL/Flask | 300-350 |
| 931144 | 250mL Ultra Yield® Flasks 50/CS - Sterile | 899423 / 899110 | 75-100 mL/Flask | 300-350 |
| 931141 | 500mL Ultra Yield® Flasks 25/CS - Sterile | 899424 / 899111 | 150-200 mL/Flask | 300-350 |
| 931138 | 1.5L Ultra Yield® Flasks 12/CS - Sterile | 899425 / 899566 | 300 mL/Flask | 300-350 |
| 931136-B | 2.5L Ultra Yield® Flasks 6/CS - Sterile | 899425 / 899566 | 500 mL/Flask | 300-400 |
| 446300 | Plasmid+® Enriched Media - Sterile | N/A | N/A | N/A |
References
- D.M. Prazeres, T. Schluep, C. Cooney, Preparative purification of supercoiled plasmid DNA using anion-exchange chromatog-raphy, J. Chromatogr. A 806 (1998) 31–45. https://doi.org/10.1016/s0021-9673(97)01254-5.
- Lionel Cupillard, Véronique Juillard, Sophie Latour, Guy Colombet, N. Cachet, S. Richard, S. Blanchard, Laurent Fischer, Impact of plasmid supercoiling on the efficacy of a rabies DNA vaccine to protect cats, Vaccine 23 (2005) 1910–1916. https://doi.org/10.1016/J.VACCINE.2004.10.018.
- 3. Huangjin Li, Huaben Bo, Jinquan Wang, Hongwei Shao, Shulin Huang, Separation of supercoiled from open circular forms of plasmid DNA, and biological activity detection, Cytotechnology 63 (2011) 7–12. https://doi. org/10.1007/s10616-010-9322.
- Christof Maucksch, Alexander Bohla, Florian Hoffmann, Martin Schleef, Manish Kumar Aneja, Markus Elfinger, Dominik Hartl, Carsten Rudolph, Transgene expression of transfected supercoiled plasmid DNA concatemers in mammalian cells, The journal of gene medicine 11 (2009) 444–453. https://doi.org/10.1002/jgm.1310.
- Arjun Dhanoya, Benjamin M. Chain, Eli Keshavarz-Moore, The impact of DNA topology on polyplex uptake and transfection efficiency in mammalian cells, Journal of Biotechnology 155 (2011) 377–386. https://doi.org/10.1016/J.JBIOTEC.2011.07.023.
- Fani Sousa, Duarte M.F. Prazeres, João A. Queiroz, Improvement of transfection efficiency by using supercoiled plasmid DNA purified with arginine affinity chromatography, The journal of gene medicine 11 (2009) 79–88. https://doi.org/10.1002/JGM.1272.

